2015 EU BPR Seminar is going to be held by CIRS and Eurofins in Hangzhou
Original Article by Ethan Zheng from CIRS
The new European Union (EU) Biocidal Products Regulation (BPR), Regulation (EU) No. 528/2012, has entered into force since September 1st 2013 and will be implemented on September 1st 2015. However, most of the Chinese suppliers and exporters who export biocidal products and/or biocide treated articles still do not recognize the regulation requirements and their obligations under BPR. For the purpose of their awareness of the obligations under BPR and within its supply Chain, CIRS and Eurofins are pleased to launch the 2015 BPR training seminar in Hangzhou.
[Download Seminar Brochure in PDF version]
Topics
1. In-depth Analysis on Authorization of Biocides Products
2. Treated Articles Compliance
3. How to Comply with Article 95 in BPR
4. Introduction of 5 Batch Analysis and Technical Equivalence under BPR
5. Introduction of GLP Lab
6. Workshop: Regulatory Compliance in Supply Chain
Time, location and language
Date: 8:00AM – 17:00PM, 14 January 2015
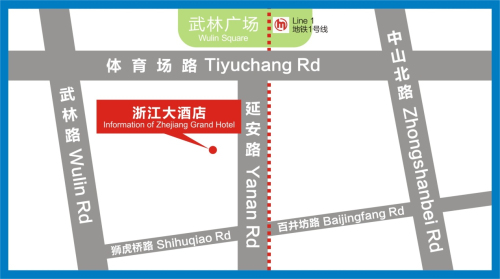
Location: Location: Zhejiang Grand Hotel, 595 Yanan Road, Hangzhou, China
Language: English/Chinese
Who shall attend?
QA Manager and Regulatory manager of Biocides manufacturers
QA Manager and Regulatory manager of Active substances manufacturers
Sale manager and Regulatory manager of Treated Articles producers
Sale manager and Technical manager of Laboratories
Speaker Profile

Agenda
Time |
Contents |
Speaker |
8:00-8:45 |
Delegates Registration |
|
8:45-9:00 |
Welcome Speech |
|
9:00-9:30 |
General introduction of BPR Compliance |
|
9:30-10:15 |
In-depth Analysis on Authorization of Biocides products |
Nicholas Jarvis |
10:15-10:55 |
Treated Articles Compliance |
David Wan |
10:55-11:20 |
Q&A |
|
11:25-13:00 |
Lunch |
|
13:00-13:55 |
How to Comply with Article 95 in BPR |
Dr. Michael Zhang |
14:00-14:55 |
Introduction of 5 Batch Analysis and Technical Equivalence under BPR |
Dr. Andreas Wais |
14:55-15:20 |
Tea Break |
|
15:20-16:00 |
Introduction of GLP Lab |
Michele Cavalleri |
16:00-17:00 |
Workshop : Regulatory Compliance in Supply Chain |
All speakers |
How to register?
The registration fee for the BPR training seminar is 1000 RMB (160 USD, 125 Euro). The fee does not cover the cost of transportation and accommodation. 20% discount is available for early birds before 31 December 2014 based on registration received.
For the information of registration, please download [registration form], fill in and send back to Mr. Ethan Zheng. If there are any questions and comments, please do not hesitate to contact us.
Mr. Ethan Zheng
Email: Ethan.zheng@cirs-group.com
Phone: +86 571 89716572
Fax: +86 571 87206533
Information of Zhejiang Grand Hotel
Contact information: +0086 571 85056666
Address: 595 Yanan Road, Hangzhou, China
Background Information
The new BPR requires that an active substance be approved by the EU and that a biocidal product be authorized before placing on the market. The BPR also requires that biocide treated articles not be placed on the EU market unless all active substances contained in the biocidal products with which they were treated or which they incorporate are approved for use in BPR. In addition to BPR regulation, more and more enterprises have paid their attentions on the information about GLP lab, 5 batch analysis and Article 95 compliance. Experts in these areas will also be invited to this seminar to share information with the attendees.
For the purpose of offering suppliers and exporters a better understanding of BPR related topics, CIRS and Eurofins are very pleased to launch this meaningful training seminar. In the case that you are doing business on BPR or if you want to know the information of these two regulations, you are very welcomed to join this seminar to share information with the speakers and other attendees.
Hosts
Hangzhou CIRS Co., Ltd. (CIRS China): Chemical Inspection & Regulation Service (CIRS) is a leading product safety and chemical management consulting firm providing valued product regulatory compliance services, tailored solutions and original information to help its clients gain competitive advantages by reducing business risks associations with regulatory affairs and removing barriers to entry.
Eurofins: Eurofins is a worldwide leader in its field. The Group offers a portfolio of more than 100,000 reliable and validated analytical methods for evaluating the safety, identity, composition, authenticity, origin, traceability and purity of biological substances and products. Through research and development and acquisitions, the Group draws on the latest developments in the field of biotechnology and analytical chemistry to offer its clients unique analytical solutions and the most comprehensive range of testing methods.

