Cosmetics and Cosmetic Ingredient Law
Updated in May 2013
In China, cosmetics and cosmetic ingredients are regulated by the following laws:
- Regulations concerning the hygiene supervision over cosmetics(1990) - main regulation;
- Detailed Rules for the Implementation of the Regulation on the Hygiene Supervision over Cosmetics(2005);
- Hygienic Standard for Cosmetics(2007)
- The Measures for the Administration of Hygiene License for Cosmetics (revised in 2010);
- Guideline for Risk Evaluation of Substances with Possibility of Safety Risk in Cosmetics(2010);
- Standard Chinese Names of International Cosmetics Ingredients Inventory (2010);
- Cosmetics Technical Requirement Standard(2011);
- Guidelines for the Registration and Evaluation of New Cosmetic Ingredient(2011);
- AQSIQ Order No. 143 of 2011 - The Administrative Measures on the Inspection, Quarantine and Supervision of Chinese Imported & Exported Cosmetics (2011)
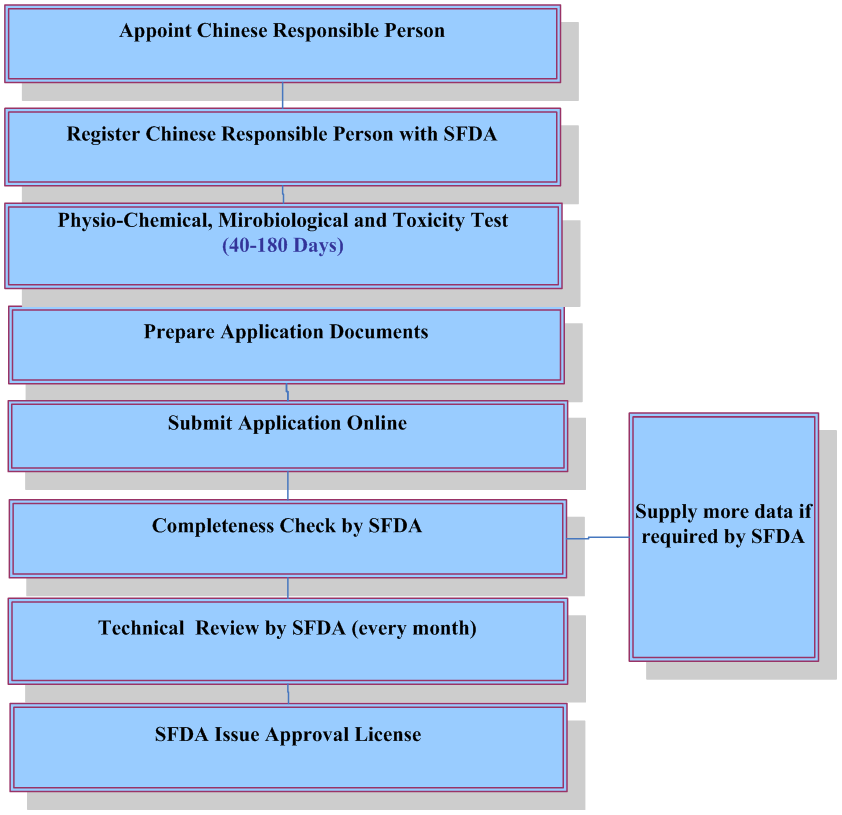
For finished cosmetics, companies who plan to place cosmetics on Chinese market must apply for and obtain hygiene license or record-keeping certificate from the China Food & Drug Administration(CFDA). Foreign companies shall appoint a Chinese responsible agent to deal with registration and obtain such certificate. Manufacturers shall also register a new cosmetic ingredient prior to using it for cosmetics production.
What is New?
20 Dec 2013, CFDA officially removes compulsory animal test for non-special use cosmetics produced in China. More info can be found here.
17 May 2013, Provincial Food & Drug Administration Authorities Will Be Responsible for Approval of Imported Ordinary Use Cosmetics in China. More info can be found here.
18-19 March 2013, CIRS to hold a workshop on invitro alternative methods for cosmetics in Guangzhou China. More info can be found here.
Definition of Cosmetics and Classification
Cosmetics are defined as daily used industrial chemicals which can be spread on the outer surface of human body (e.g. skin, hairs, nails. lips etc) for the purpose of cleaning, deodorizing, providing skin care, beauty and make – up, by way of smearing, spraying or other similar means.
Imported cosmetics are divided into two classes: ordinary cosmetics and special use cosmetics. Each class requires different type of license from CFDA. Please note that imported ordinary use cosmetics are approved by food & drug administration authorities at provinical level while imported special use cosmetics are approved by CFDA in Beijing.
| Type of Product | Required License | Who Shall Apply? |
|---|---|---|
Ordinary cosmetics hair care, nail care, skin care, perfumes and make-up |
Record-keeping Certificate
|
A Chinese responsible agent appointed by a foreign manufacturer |
Special use cosmetics products for hair growth, hair dye, hair perm, hair removal, breast shaping, fitness, deodorizing, spots removal and sun block; Cosmetics with skin-whitening and skin pigmentation reduction claims will be classified as special use cosmetics (anti-freckle category) from 16th Dec 2013. |
Hygiene License
(More expensive and time-consuming)
|
|
New Cosmetic Ingredient |
Hygiene License |
Requirements of Ingredients in Cosmetics
The Hygienic Standard for Cosmetics published by the Ministry of Health in 2007 has banned over 1200 chemicals in cosmetics and restricted the use of 73 chemicals, 56 preservatives, 156 colorants, 28 sun block agents and 93 dyes in cosmetics. Before companies apply for hygiene license or record-keeping certificate, companies shall make sure their formula meets this hygienic standard.
Companies shall also check if there is any new ingredient in their product. A new ingredient that is not currently listed on the Inventory of Existing Cosmetic Ingredients in China (IECIC) also requires registration with SFDA. However, a new ingredient might not require registration if there is proof that the ingredient has been used in approved cosmetics in China before. It can be very difficult and expensive to register a new ingredient. More info about registration of new cosmetic ingredient can be found here.
What is New!
4 Dec 2012, China SFDA to Revise Hygienic Standards for Cosmetics and Lower Pb and As Level in Cosmetics. For more info, please click here.
We can help you search both IECIC(2003) & IECIC(2014 draft) & Hygienic Standard for Cosmetics, determine if an ingredient is new or not and identifiy maximum allowable use level if there is any restriction in IECIC & hygienic standard for cosmetics(2007) in China. This service costs RMB 100-300 per ingredient depending on the number of cosmetic ingredients to be searched. Please note that the first ingredient search is free of charge. You can also request a consolidated list of IECIC(2003, Chinese) and IECIC(2014 Draft, English version) free of charge by contacting service@cirs-reach.com
Application of Hygiene License or Record-keeping Certificate
The following documents are required for application of hygiene license or record keeping certificate:
- Application form for license;
- Product ingredients;
- Effective components, evidence of use and inspection methods;
- Manufacturing technique and diagram;
- Product quality standard;
- Testing report from a cosmetics testing institution approved by the SFDA and related materials;
- Product package (sales package & product label);
- Certified document for production and sales in the manufacturing country (region);
- The statement on related problems of ‘Mad Cow Disease’;(new)
- Power of attorney, if responsible person has been appointed;
- Some other documents that may be helpful for inspection;
Note: Some documents are not required for ordinary use cosmetics.
Testing Requirements
According to the official implementation of the national standard: Procedures and methods of safety evaluation for cosmetics (GB 7919-87), some of the following testing items are required depending on the types of finished cosmetics. Please contact April.guo@cirs-reach.com if you would like to know what tests are required for your products.- Physiochemical and microbiological testing;
- Acute oral toxicity and acute dermal toxicity;
- Acute dermal irritation and acute eye irritation;
- Dermal sensitization;
- Dermal photo-toxicity test & dermal photosensitivity;
- Sub-chronic oral toxicity and dermal toxicity;
- Teratogenicity test;
- Authenticity test;
- Chronic toxicity and carcinogenic test;
- Safety evaluation of using tests of cosmetics on human body;
Labeling Requirements
According to the official implementation of the national standard: Instruction for use of consumer products— general labeling for cosmetics (GB5296.3-2008), the following information, in Chinese, needs to appear on a label for cosmetics:- Product name;
- Name and address of the manufacturer;
- Net content;
- Product ingredients;
- Shelf life;
- The code of manufacture license and product standard;
- The code of hygiene license or record-keeping certificate;
- Safety statement and guidance on uses;
In case of imported cosmetics, country of origin and the name and address of the distributor in China shall also be given on the label.
CIQ Inspection of Imported Cosmetics
Even though CIQ labels will no longer be required for imported cosmetics from 1 Feb 2012, CIQ's inspection is mandatory. For cosmetics imported to China for the first time, Chinese importer needs to provide the following documents when applying for an inspection from CIQ:
- A self-declaration letter stating that the imported cosmetic product complies with relevant Chinese laws and the normal use of the product will not cause any harm to human health;
- Product formula;
- Hygiene license or record-keeping certificate;
- For cosmetics exempted from hygiene license or record-keeping requirement, the following documents are required:
- Safety evaluation report issued by the qualified institutions for substances of potential safety risks; and
- Documentation that permits the production and distribution of the imported cosmetics in the country of production or a Certificate of Country of Origin;
- Sample labels in Chinese, product labels in the original language and the translated text in Chinese;
- Information on the product name, volume/weight, specifications, country of origin, batch number, expiry date (production date and shelf life), target market, and information about packaging company;
- Other documentation required by AQSIQ.
Please note that the documents required for bringing non-trade cosmetics(for example, product samples, R&D samples) into China are different. More info can be found here.
How Much Does Application Cost Per Product?
The total costs of application consist of three parts: Testing fee, risk evaluation fee charged by approved inspection authorities and consulting fee. The total costs vary among different service providers, quoted prices in various inspection authorities, and test items for the distinct usages of cosmetics.
Generally speaking, the total application cost of record-keeping certificate for imported ordinary cosmetics is between 10,000 and 30,000 RMB per product.
The total application cost of hygiene license for imported specific use cosmetics is between 20,000 and 60,000 RMB per product.
The total application cost of hygiene license for new cosmetic ingredient is between 80000 and 100000 RMB per ingredient.
Please note the above cost estimation does not include risk evaluation fee and human body test.
Our Services
Previously set up by China Inspection and Quarantine Bureau(CIQ), CIRS has strong presence in China and close links with regulatory authorities in China, especially CIQ. CIRS China can act as the Chinese responsible agent, review your ingredients, coordinate testing, prepare application materials and obtain hygiene license or record-keeping certificate and CIQ labels for your cosmetics products.
If you wish to avail of our integrated compliance solutions for the entry of cosmetic products into China, please don't hesitate to consult us.
Contact Us
Ms April Guo, CIRS China
Specialized field: application for hygiene License, record-keeping certificate for imported cosmetics, safety evaluation of cosmetics and cosmetic ingredients, standard labeling for cosmetics, SDFA registration, CIQ label, etc.
- Ms April Guo, China Office
11F Building 1, Dongguan Hi-Tech Park, 1288 Chunbo Road, Binjiang District, Hangzhou 310052, China
Tel: +86-571 8720 6555 | Fax: +86-571 8720 6533
Email: april.guo@cirs-reach.com

Related
- Guidance on Exporting Cosmetics to China[pdf] 2016
- Guidance on Exporting Cosmetics to China[pdf] 2012
- SFDA Registration of New Cosmetic Ingredient
- Inventory of Existing Cosmetics Ingredients China (IECIC)
- Presentation: Application of SFDA license and CIQ label for imported cosmetics in China by Ms April Guo
- Download Brochure
- China IECSC