
REACH Registration - Joint Submission
To minimize animal testing and data fee for each registrant, REACH registration encourages data sharing among all registrants by the joint submission of registration data to ECHA. The principle is “one substance, one registration”.
During joint submission, the lead registrant or REACH consortium will do most of the work such as data collection, development of technical dossier and Chemical Safety Report (CSR), and submission of joint registration dossier to ECHA. Other co-registrants only need to pay the lead registrant or consortium a fee to refer to the joint registration dossier and then prepare the individual part of the registration dossier in IUCLID 5.
Registrants will have two options to complete registration as a member of joint submission: Purchase a letter of access or join REACH consortium. Both options allow registrants to refer to the joint registration dossier prepared by lead registrant or REACH consortium. Generally, a letter of access is cheaper because it does not grant ownership of data.
REACH Registration Process for Phase-in Substances
- After pre-registration, all pre-registrants of the same substance are put into one Substance Information Exchange Forum(SIEF), in which members exchange information and share data;
- SIEF facilitator conducts survey on registration intention, substance sameness and data availability;
- Lead registrant is elected by all SIEF members (in many cases, LR is a large company or a member of a consortium);
- Lead registrant collects data, prepares joint registration dossier (technical dossier and/or Chemical Safety Report) and submits the dossier to ECHA in REACH-IT;
- Co-registrants pay lead registrant a fee(buy a letter of access or join REACH consortium) to refer to the common part of registration dossier and receive the name of joint submission and a security token from lead registrant;
- Co-registrants confirm membership of joint submission in REACH-IT, complete the individual part of registration dossier in IUCLID 5 and submit it to ECHA;
- Co-registrants pay ECHA administrative fee and receive a registration number;
In rare cases, joint registration dossier might not include Chemical Safety Report or not cover certain uses. Member registrants need to prepare their own Chemical Safety Report correspondingly.
For more information about joint submission process (REACH-IT, LEO, IUCLID 5) and how to prepare the individual part of registration dossier in IUCLID 5, please click CIRS's presentation on joint submission process and reach registration dossier preparation[pdf, 2.4MB].
REACH Registration Cost Estimation
The total registration costs mainly consist of three parts:
- Data fees paid to lead registrant or Consortium to purchase letter of access to refer to the common parts of registration dossier. The fee is dependent on annual tonnage. Data fee varies from substance to substance. It is fixed by consortium or lead registrant. It is also tonnage based. For example, the letter of access for Iron costs 2500 Euros for 1000t/y+. The letter of access for many petrochemicals cost 10,000 Euros per substance.
- Consultancy fees paid to CIRS to advise the whole registration process and prepare the individual part of registration dossier in iuclid 5. This service fee is fixed by CIRS and it varies from substance to substance with the maximum charge of 4,000 Euros per substance. The price is lower for lower tonnage band. It includes all the work that needs to be done to acquire a registration number (no hidden fees).
- ECHA fees paid to European Chemical Agency. The fee is dependent on the annual tonnage of the substance and company size. Small and medium-sized enterprises (SME) enjoy discounts on ECHA fees (Euros).
Tonnage |
Normal |
Medium |
Small |
Micro |
>1000t/y |
23,250 |
16,235 |
9,300 |
2,325 |
100t-1000t/y |
8,625 |
6,038 |
3,450 |
863 |
10t-100t/y |
3,255 |
2,258 |
1,290 |
323 |
1t-10t/y |
1,200 |
840 |
480 |
120 |
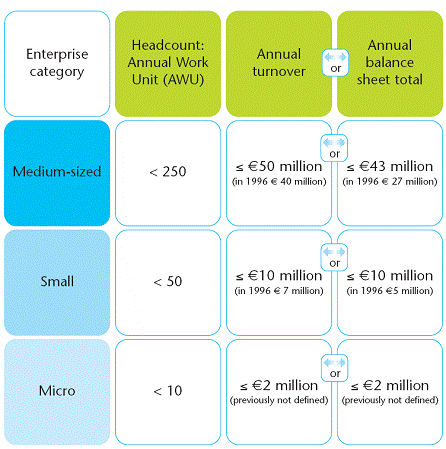
SME criteria is listed as below:
Note: Besides above fees, member registrant also needs to do some analytical tests such as HPLC, MS, IR, and NMR etc to confirm substance identity. This might cost extra 800 Euros. The whole registration process takes 2~4 months.
The whole registration process takes 2~4 months.
Our REACH Registration Services
Our full-range REACH registration services will provide end-to-end solutions to REACH registration. We have helped hundreds of non-EU firms and EU companies acquire over 180 REACH registration numbers up to date. The reasons to choose us include:
- Extensive substance specific registration experience;
- Broad communications with SIEF/Consortium;
- No hourly rates and hidden charges;
- Success guaranteed;
- Free regulatory updates and free consultations;
Please don't hesitate to contact us if you would like to find out whether you need to do reach registration or not and how much it might cost you. Our initial consultations are free.
Contact
- Europe Office
Unit 1 Ardee Business Park, Hale Street, Ardee, Co. Louth, Ireland
Tel : +353 41 9806 916 | Fax : +353 41 9806 999
Email: louisen@cirs-reach.com


_will_be_held_in_Shanghai.jpg)