
PPORD Notification
What is PPORD notification?
Article 3(22) of REACH outlines that ‘product and process orientated research and development'(PPORD) means any scientific development related to product development or the further development of a substance, on its own, in preparations or in articles in the course of which pilot plant or production trials are used to develop the productions process and/or to test the fields of application.
Substances used for the purpose of PPORD will be exempted from REACH registration for 5 years. However, companies shall submit PPORD notifications to ECHA.
PPORD notification: what information is required?
Article 9(2) of REACH outlines that the following information is required for a PPORD notification:
- the identity of the manufacturer or importer;
- the identity of the substance;
- the classification of the substance;
- the estimated quantities;
- the list of customers (including names and addresses).
PPORD notification: typical process
To complete PPORD notification, a dossier needs to be made by software called IUCLID 5 and submitted to the European Chemical Agency for evaluation. In order to complete this process the following typical steps need to be carried out:
- Create the legal entities of the PPORD notifier and its customers;
- Create the reference substances related to the PPORD substances;
- Create the substance dataset for the PPORD substance;
- Create the PPORD notification dossier;
- Export the PPORD dossier from IUCLID 5 and submit dossier to ECHA via REACH-IT;
- ECHA will undertake a completeness check of the dossier;
- ECHA provides a notification number once PPORD fee has been received and dossier has passed TCC (technical completeness check).
PPROD notification: how much does it cost?
The total registration costs mainly consist of twoparts:
- Consultancy fees paid to consultancy firms to advise the whole PPORD notification process, gather data and prepare notification dossier in iuclid 5. CIRS usually charges 1,000 to 3,000 Euros for this service depending on the number of substances and the complexity of the substance.
- ECHA fees paid to European Chemical Agency. The fee is dependent on the size of the company. Small and medium-sized enterprises (SME) enjoy discounts on ECHA fees (Euros).
|
Normal |
Medium |
Small |
Micro |
ECHA Fee |
500 |
350 |
200 |
50 |
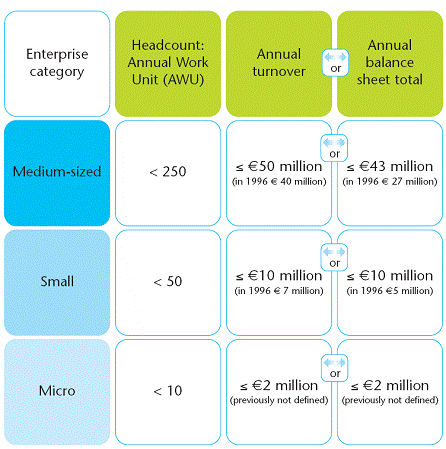
SME criteria is listed as below:
About CIRS
CIRS a leading provider of comprehensive chemical compliance services for companies doing businesses in/with EU and China with a strong focus on chemical compliance.
With a strong presence in EU and China, CIRS has provided cost-effective regulatory support to over 3,000 companies while doing businesses in both the EU and China.
CIRS is the largest REACH only representative in the world. Since 2007, we have:
- pre-registered over 10,000 substances;
- acted as only representative for over 2,400 non-EU companies;
- served clients in more than 25 countries;
- registered over 145 substances to date;
- prepared over 1000 REACH SDS and CLP labels to date;
- submitted over 500 C&L Notifications to date;
CIRS is a recommended service provider by China Inspection and Quarantine Bureau, the US Mission to the EU and IDA. CIRS is also a member of Helsinki REACH Centre.
Contact
- Europe Office
Unit 1 Ardee Business Park, Hale Street, Ardee, Co. Louth, Ireland
Tel : +353 41 9806 916 | Fax : +353 41 9806 999
Email: service@cirs-reach.com


_will_be_held_in_Shanghai.jpg)