The Administrative Measures on Health Food Registration and Filing came into force on 1 July 2016. In the early stage of the implementation of the Measures, the progress of health food registration was relatively slow due to the changes of relevant policies. Only 10 products were approved for the registration in 2018.
Fortunately, it seems to be smooth since 2019. There were 342 products obtaining the health food registration approval in 2019 and 715 products obtaining the health food registration approval in 2020.
In order to help enterprises have an overview of this kind of products in China, CIRS counted the data of the approved health food registration products in 2020, and made an analysis for your reference.
1. The Number of Health Food Approved for Registration in Every Month of 2020
715 health foods have been approved for registration in 2020. The number of approved products in each month is demonstrated in Fig.1.
As you can see in Fig.1, the issuance of new product registration approval is mainly concentrated between January and May, and the issuance of other approval (registration renewal, registration alteration, technology transfer) is mainly concentrated between June and December. (Note: the final information is subject to the special food information query platform)
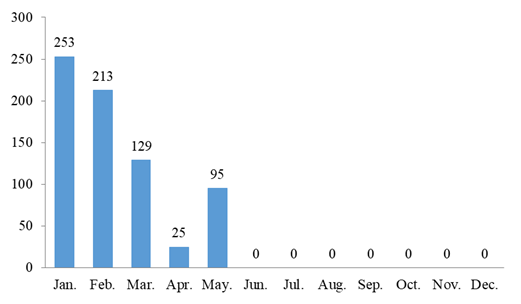
Fig.1 The Number of Approved Products in every Month of 2020
2. The Acceptance Time (as year) of the Approved Products
Among the 715 approved products, 577 products have been officially published. The 577 products have been accepted by the former CFDA in the past few years (mainly from 2013 to 2015), and was mainly in 2014, with the number of 363, accounting for 62.91% of the total, followed by 2015 with the number of 141, accounting for 24.44% of the total. The details are as the Fig.2 below:
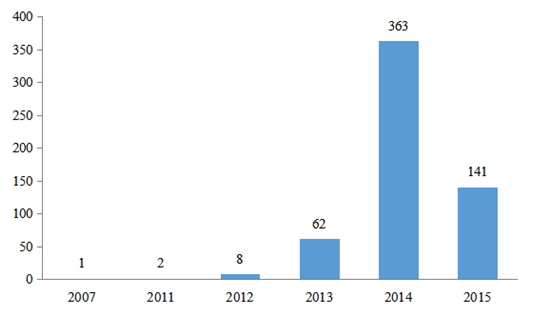
Fig.2 The Number of the Products Accepted in Different Years
3. The Number of Approved Products in Different Regions
The number of approved products is varied in different regions (provinces, municipalities and autonomous regions) in China. Beijing has the most, with the number of 131, accounting for 18.32% of the total, followed by Guangdong, with the number of 121, accounting for 16.92% of the total.
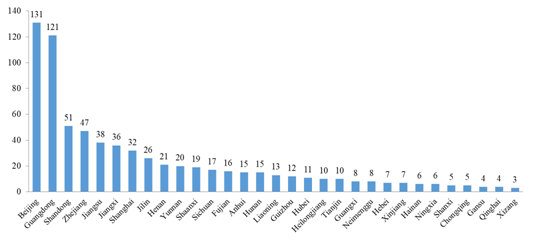
Fig.3 The Number of Approved Products in Different Regions
4. Enterprises Obtaining Health Food Registration Certificates
The 715 approved products come from 492 health food enterprises. Among them, Beijing Century Health Care Pharmaceutical Technology Co., Ltd. has got the largest number of registration certificates (19), followed by Beijing Dingweifen Health Technology Co., Ltd. with the number of 11.
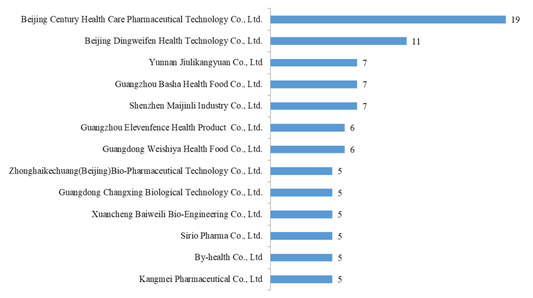
Fig.4 Top 13 Enterprises in Obtaining Health Food Registration Certificates in 2020
5. The Number of Approved Products with Different Dosage Forms
The dosage forms of approved products include capsule, tablet, soft capsule, powder, granule, oral liquid, beverage, wine, tea, pill, extract, candy, liquid milk, etc., and are mainly capsules, with the number of 218, accounting for 30.49% of the total, followed by tablets, soft capsules and powder with the number of 131, 128 and 82, accounting for 18.32%, 17.90% and 11.47% of the total, respectively.
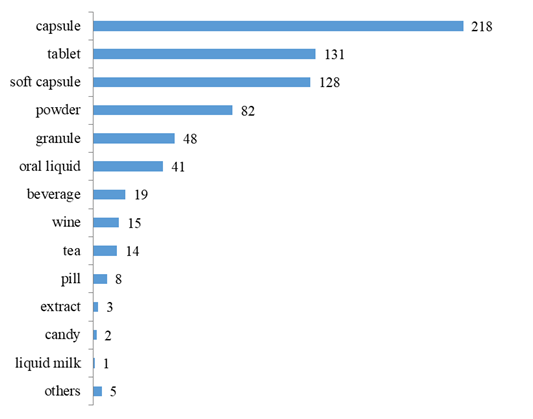
Fig.5 The Number of Approved Products with Different Dosage Forms
6. The Number of Approved Products with Different Health Functions
Among the 715 approved products, the health functions of 660 products have been officially published, include:
1) 610 products with single health function, which is mainly enhancing immune, with the number of 369, accounting for 60.49% of the 610 products;
2) 43 products with two health functions, which are mainly enhancing immune and alleviating physical fatigue, with the number of 18, accounting for 41.86% of the 43 products;
3) 7 products with the functions of supplementing vitamins/minerals.
The health functions of the remaining 55 products have not been published yet, while it could be speculated from their raw materials that, the health function is mainly enhancing immune.
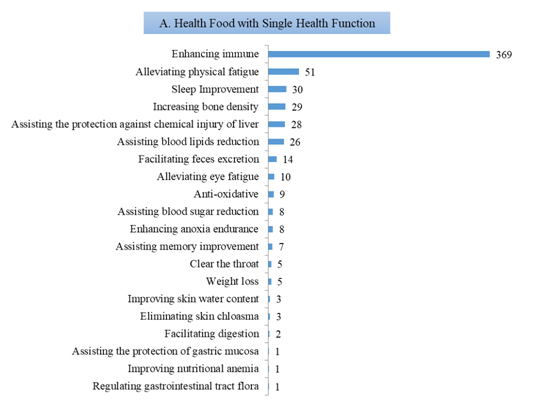
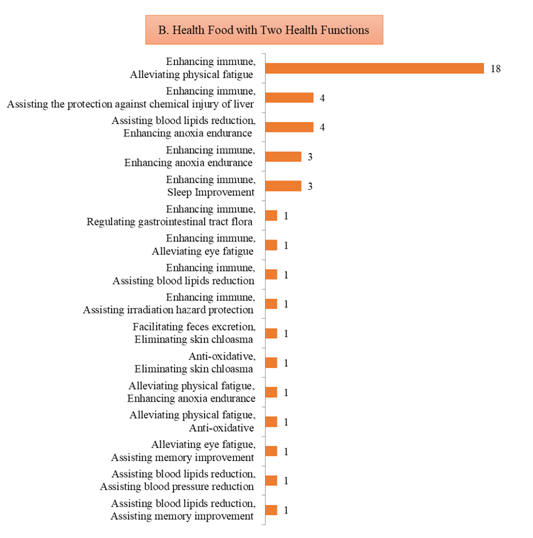
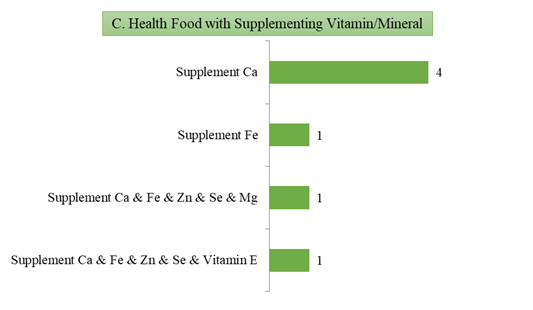
Fig.6 The Number of Approved Products with Different Health Functions
7. CIRS Comments
Due to the change of health food registration policies and the institutional reform, the registration work of health food has been stagnant. Compared with 2018 and 2019, much more progress has been made in 2020. The number of health food registered products reached 715 in 2020, far more than that in 2019, which indicates the improvement in the implementation of the new health food regulations. Meanwhile, the products that have been backlogged for several years are solved one after another in 2020. It is believed that the registration work on health food will also be carried out smoothly in 2021.
If you have any needs or questions, please contact us at service@cirs-group.com.
Source
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.

