Background
In accordance with Food Safety Law of the People's Republic of China, companies that plan to use new food raw materials to manufacture food products shall submit the related safety assessment material of the new food raw material to National Health Commission of the People's Republic of China (former NHFPC).
New food raw material in China
New food raw material refers to the following substances that have no traditional eating habits in China, including:
- Animals, plants and microorganisms;
- Components extracted from animals, plants and microorganisms;
- Food ingredients that have changed in the original structure;
- Other
newly developed food ingredients.
New food raw materials should have the characteristics of food raw materials and conform to the requirements of nutritive. It shall be non-toxic, harmless, and not cause any acute, subacute, chronic or other potential hazards to human body.
Relevant laws and regulations

Registration dossier
1) Application form;
2) Development report of new food raw material;
3) Safety assessment materials;
4) Manufacturing technique;
5) Relevant standards (including safety requirements, qualification specification requirements and test methods, etc.);
6) Label and specification;
7) Research situation of domestic and overseas, and related-safety assessment materials;
8) Power of attorney (when the registration application run by entrusted agencies);
9) Other materials that contribute to safety review.
Note: One piece of minimum packing sample or 30 g raw material shall be provided.
For imported new food raw material, besides the above documents, the following supplementary documents also should be submitted:
10) Certifying documents issued by government authorities or industry associations in the exporting country (region) of origin, proving that the new food raw material is allowed to manufacture or sell in the country (region).
11) Certifying documents issued by government authorities or industry associations in the producing country (region) of origin, proving that the new food raw material manufacturer has been examined or certificated.
Test requirements
- Composition analysis report: the test results and methods of main components and possible harmful components.
- Hygienic inspection report: the test results and methods of pollutants and microorganism.
- Toxicology safety assessment documents:
Conditions | Test requirements in principle |
No traditional eating habit of domestic and overseas (excluding microorganism) | Acute oral toxicity test; Three genetic toxicity tests; 90-day oral toxicity test; Teratogenic and reproductive toxicity tests; Chronic toxicity and carcinogenicity tests; Metabolic test. |
Only have traditional eating habit in several foreign countries or local areas (excluding microorganism) | Acute oral toxicity test; Three genetic toxicity tests; 90-day oral toxicity test; Teratogenic and reproductive toxicity tests. |
Widely approved to use in many countries (excluding microorganism) | Acute oral toxicity test; Three genetic toxicity tests; 28-day oral toxicity test. |
Microorganism that without traditional eating habit of domestic and overseas | Acute oral toxicity test/Pathogenicity test; Three genetic toxicity tests; 90-day oral toxicity test; Teratogenic and reproductive toxicity tests. |
Microorganism that only have traditional eating habit in several foreign countries or local areas | Acute oral toxicity test/Pathogenicity test; Three genetic toxicity tests; 90-day oral toxicity test. |
Microorganism that have been approved to use in many countries | Acute oral toxicity test/Pathogenicity test; Two genetic toxicity tests. |
- Microbial resistance test report and toxigenicity test report: applicable to microbial new food raw materials.
- Safety
assessment: including hazard factor identification, hazard characterization,
exposure assessment and risk characterization.
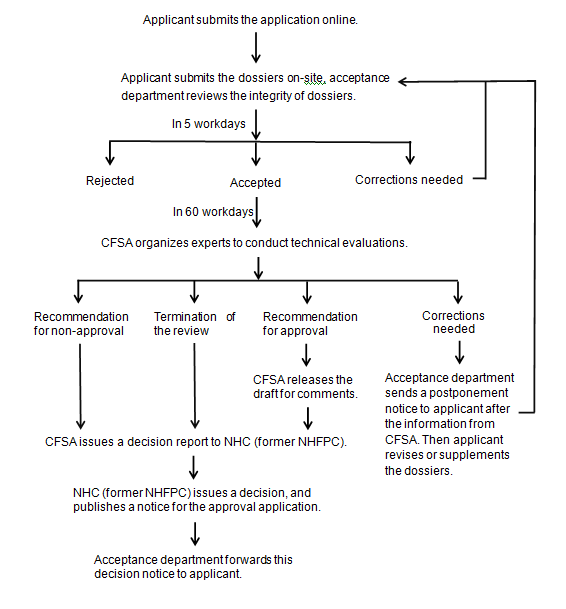
Work process of administrative approval
NHC: National Health Commission of the People's Republic of China
NHFPC: The National Health and Family Planning Commission
CFSA: China National Center for Food Safety Risk Assessment
Our services
1. New food raw material registration
- Registration dossier preparation and submission;
- Foreign documents translation and notarization;
- Tests arrangements and monitoring;
- Project progress tracking;
- Attend the expert appraisal conference and assist enterprise answer the technical questions;
2. Training on new food raw material registration
If you have any other questions, please feel free to contact us at service@cirs-group.com
