Since the" Measures for the Administration of Registration and Filing of Health Food" officially implemented, the registration and filing of health food in China have been developing steadily and normatively. As of June 30, 2021, 43 announcements about the registered products were issued by Food Evaluation Center of State Administration for Market Regulation, involving 932 products.
However, due to the little information disclosed in these announcements, the 932 products can't be classified accurately. CIRS counted the data from Special Food Information Query Platform of State Administration for Market Regulation (hereinafter called as “the system”), to get the following information.
1. The general situation of health food approved for registration in the first half of 2021
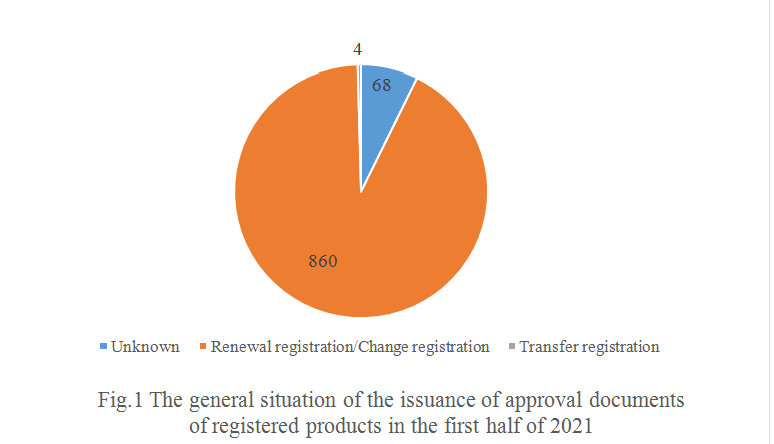
Based on the information currently recorded in the system, most of the 932 products are renewal registration and/or change registration. And 4 products are presumed to be transfer registration.
As shown in Fig.1, 860 products are presumed to be renewal registration and change registration, accounting for 92.27% of the total. In addition, 68 products, accounting for 7.30% of the total, can't find relevant information in the system, CIRS speculates that newly registered products may exist among these products.
2. Changes of the product names
Among the 932 approved products, 260 of them changed their names. According to " the Guidelines for Naming of Health Food (2019 version)" issued by the SAMR (hereinafter referred to as "the Guide"), the name of health food is composed of trade name, general name and attribute name.
2.1 Change the general name
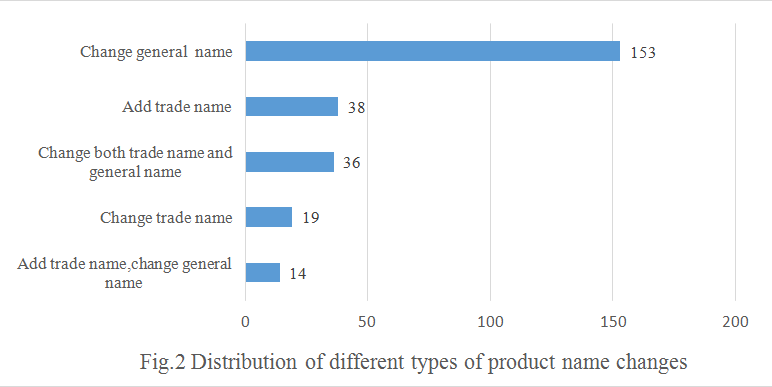
The general name is the name that indicates the main material of the product, etc. "The Guide" requires that general name should be named as the full or abbreviation mane of main raw materials. When a health food is made of several ingredients, in principle, the name shall not be contain more than 3 kinds of raw material name. As Fig.2 to see, 153 products changed their general names, accounting for 58.9% of the total number of product name changes.
For example, a health food called "Duomeijiao brand hawthorn, medlar, angelica, ginseng, deer antler, donkey-hide gelatin, iron granules "before changing name. Based on "the Guide", its name was changed to Duomeijiao brand ginseng, deer antler, donkey-hide gelatin granules.
2.2 Add the trade name
The registered or non-registered trademarks in mainland of China can be used as trademark name. In the past, some health food didn't have trade name, so that needed to add according to the new regulation. As shown in Fig.2, 38 products have added the trade name.
For example, the "Baihekang brand DHA Algae Oil and Flaxseed Oil Soft Capsule" registered by Weihai Baihe Biotechnology Co., Ltd., was called "DHA algal oil flaxseed oil soft capsule" before. The change was due to the lack of trade name.
2.3 Change both trade name and general name
Moreover, some of the products changed both trade name and general name. For example, a product of Guangzhou Jianyuan Biological Technology Co., Ltd, was named as "Jianyuan Yiyun Tablet" before, and the name is changed into "Guangshanyuan brand L-carnitine Chitosan Tablet".
3. CIRS comments
The data of the first half of 2021 for registration of health food approval shows that few new products are approved for registrations in recent half, and most of the approved products are renewal registration and change registration.
SAMR issued the "Guidelines for Naming of Health Food (2019 version) " to standardize the naming of health foods. The names of all health food shall comply with this regulation. More detailed interpretation of the Guide, please click "Key Points of Guidelines for Naming of Health Food (2019 Version) ".
The data source:Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements. Because the Special Food Information Query Platform lag behind in information release, the data in this article is for reference only.
If you have any needs or questions, please contact us at service@cirs-group.com.

