CIRS Group Korea was issued a license for Cosmetic Responsible Sales Business on February 27th 2019. As obtaining a Cosmetic Responsible Seller is mandatory for exportation of cosmetics to Korea, overseas cosmetic companies will be able to export cosmetics through CIRS Group Korea. Below is a flow chart of procedure of cosmetic importation in Korea, required documents for it.
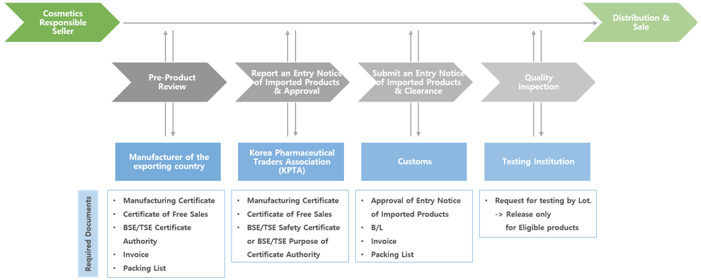
Procedure of Cosmetics importation in Korea and required documents
Obligations of Responsible Seller and CIRS Group Korea Service

When importing and distribution cosmetics, under the provisions of Cosmetics Act, the overseas companies must appoint a Cosmetics Responsible Seller who has already registered a Cosmetics Responsible Sales Business license. The Cosmetics Responsible seller is provided relevant dossier from exporters such as results of production or import cosmetics and a list of raw materials to complete an Entry Notice of Imported Products to register and report it as the Electronic Data Interchange (EDI) to the Korea Pharmaceutical Traders Association (KPTA). After approval from KPTA of an Entry Notice of Imported Products, Cosmetics Responsible Seller shall file an import declaration and be issued an import declaration certificate. Then, the products shall be tested in the testing institution contracted with the Cosmetics Responsible Seller and released only for eligible products with Korean label.
The above details are the import procedure of general cosmetics. For functional cosmetics, only those products that have obtained permission from the Ministry of Food and Drug Safety (MFDS) after functional examination before reporting an Entry Notice of Imported Products can be imported.
Below is the definition and classification of cosmetics under the provisions of Cosmetics Act in Korea.
Definition and Classification of Cosmetics in Korea

CIRS Group Korea’s service content is as follows:

CIRS Group Korea will devote itself to providing Cosmetics Responsible Seller, pre-review the suitability of importation, review of label, application and approval of an Entry Notice of Imported Products, preparation of an import declaration and payment of tariff, and quality inspection to successfully register export cosmetics in Korea.
If you have any other needs or questions, please contact us at service@cirs-group.com.
Certificate of Cosmetics manufacturing and sales business (KR)
Certificate of Cosmetics manufacturing and sales business (EN)

