According to the New Food Safety Law of the People's Republic of China and the Administrative Permission Law of the People's Republic of China, CFDA issued the Administrative Measures of Dietary Supplement Registration and Notification (draft version for public comments) on 28 July 2015, in an attempt to regulate the registration and notification for dietary supplement. The public can present opinions and advice for The Measure before 28 August this year. CIRS speculated that the final version of the Administrative Measure of Dietary Supplement Registration and Notification will have slight modification compared with the draft version, and will be issued before 1 October 2015. It is said that there would be a transitional period. At the same time, the Administrative Measure of Dietary Supplement Registration (trial version issued on 30 April 2005) will be replaced.
Compared with the Measures issued in 2005, the new Regulation has a tremendous change, and will impose more stringent management on dietary supplement registration.
Due to the New Regulation, what kind of dietary supplement should be registered?
The dietary supplement of which raw materials are not listed in the Catalogue of Raw Materials of Dietary Supplement.
The dietary supplement which is imported into China for the first time.
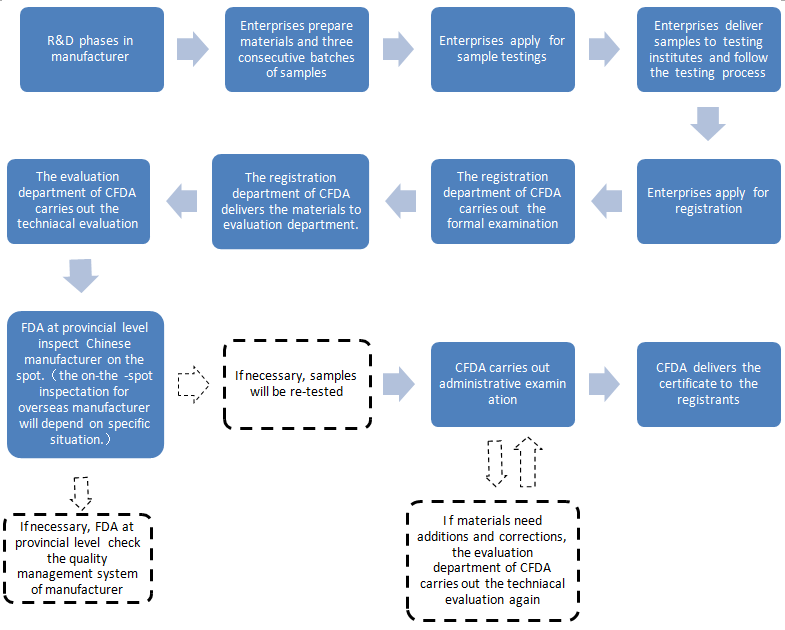
The new procedures of the dietary supplement registration
The change of required materials of dietary supplement registration
NO. | Registration required materials | The differences between the law in 2015 and in 2005, and relevant comments. |
1 | The application form of dietary supplement registration | No big change. |
2 | The originals and copies of legal registration document of registrants. | No big change. |
3 | Retrieval information (retrieved from CFDA government website database) proving that the generic names of the dietary supplement being applied don’t overlap with the names of products already registered | No big change. |
4 | R&D materials of products | R&D materials of products were replaced as more completed and scientific R&D data reports. This change demonstrated that Chinese governments inclined to pay more attention to the safety, scientificity and functionality of product formula. |
5 | Product formula materials | The new law requires that the product formula materials should include the qualification and survey report of raw materials. CIRS advises that foreign manufacturer of dietary supplement should be more familiar with the standards of quality, variety and dosage of raw materials that formulated by Chinese governments. |
6 | Product process materials | Product process materials should include the variety, name, quality standards and choosing basis of packing material of direct contact product. This change showed that foreign manufacturer of dietary supplement should research the product quality stability in shelf life. |
7 | Materials of product safety and function evaluation. | Materials of product safety and function evaluation were added the consumption evaluation, the specific methods and detailed description of reevaluation test of product safety and healthcare function (pretreatment method and inspection periods of samples should be clear) and related research materials. This change showed that Chinese government will be more stringent to the testing methods and result of product toxicological security and function. |
8 | Technical requirement of products. | The requirements of product technologies were added that the key process control points, standard and source of raw materials, detailed description and related research materials. This change showed that Chinese governments pay more attention to the manufacturer’s research on the product process, quality standards, and examination to raw materials supplier. |
9 | Product labels and manual sample | The new guideline of dietary supplement labels has many changes, which showed that Chinese governments will be stricter to the requirements of the unsuitable crowds, attentions and label formats of dietary supplement. |
10 | Symposium of comprehensive analysis on product safety, healthcare functions and quality controllability and related scientific basis. | Symposium was the newly added item in the Measure. From now on, the registrant of dietary supplement need to generalize and summarize the toxicology testing report, healthcare functions report and product R&D report. |
11 | Three unopened minimum-packing samples in shelf life. | The previous law needs only two samples. The new law requires three. |
12 | Other materials that may help product registration. | No big change. |
NO. | The additional materials that required for imported dietary supplement registration | The differences between the law in 2015 and in 2005, and relevant comments. |
1 | The certificates which demonstrate that the registrant is the legal possessor of the product. The certificates should be issued by the countries or regions where manufacturer belong to and have the valid period on it. | The new law added the report of safety situation of selling and consumption. If a product has a good sale in overseas and has no any safety problem, it would be much helpful to its registration in China. |
2 | The certificate which certifies that this product has been on sale for more than 1 year. This certificate should also be issued by original country. | |
3 | The certificate which certifies that manufacturer meets the local quality management standards. This certificate should be issued by original country and have the valid period on it. | |
4 | The analyzing report about the sales and the consumption situation in original country. | |
5 | The package, labels and manual samples of the product in original country or region. | |
6 | The product-related standards from original country or international organizations. | |
7 | Three conductive batches of on sale products or specially treated samples which used in review testing. The number is triple to the quantity needed by testing. | |
Notes: All foreign language materials provided by manufacturers need to be translated into Chinese. And Chinese document should be notarized by Chinese notary department. The certificates provided by foreign institutes should be notarized by local notary department and need to be confirmed by Chinese Embassy. The product quality standards (Chinese version) have to be in line with the format of Chinese quality standards of dietary supplement. | ||
The Administrative Measures of Dietary Supplement Registration and Notification (draft version for public comments) demonstrated that Chinese government will be more stringent to the requirements of safety and function of dietary supplement. Therefore, only deep analyze this guidance, can manufacturers enter into broad Chinese market with stronger competitiveness and lower cost.
If you have any questions and comments on the regulations, please contact us as follows:
11F Building 1, Dongguan Hi-Tech Park, 288 Qiuyi Road, Binjiang District, Hangzhou 310052, China
Tel: 0086 571 8720 6574
Fax: 0086 571 8720 6533
Email: service@cirs-group.com (EN), Info@cirs-group.com (CN)

