Updated on 3 Jul. 2020
Background
In accordance with Article 30 of the Toxic and Concerned Chemical Substances Control Act (TCCSCA, revised in 2019), enterprises that manufacture or import certain quantities of existing chemical substances each year shall submit the registration dossier of chemical substance to the competent authority before the specified deadline.
In accordance with Article 16 of the Regulations of New and Existing Chemical Substances Registration (Sub-regulation of TCCSCA, revised in 2019), the competent authority may, by stages, designate the lists of existing chemical substances subject to standard registration, including the names of the chemical substances, quantity thresholds and the deadlines for registration, based on the circumstances of the phase 1 registration of existing chemical substances.
Note: Annex 6 covers the designated list of existing chemical substances subject to standard registration (106 substances), the quantity thresholds and the deadlines for registration. The list can be searched via CIRS's ChemRadar.
Annex 7 covers the information requirements of existing chemical substances standard registration
Who Shall Register
- Manufacturers of the designated existing substances;
- Importers of the designated existing substances;
The Registrants must be the legal entities or natural person in Taiwan. Registrants may entrust a representative to complete registration, and representatives must be the legal entities or natural person in Taiwan.
Note: 1. Taiwan importers refer to the taxpayers in the Customs Declaration Form; 2. When representatives submit the application, they shall also provide a notarized appointment letter; 3. If CBI protection is required, importers in Taiwan may entrust an representative to apply for registration. Registrants (importers in Taiwan) cannot inquire on the confidential information.
Existing Substance Standard Registration is Required
Existing chemical substance standard registration is required when substances satisfy the following three conditions:
- Substances with Phase I Standard Registration numbers;
- Substances have been listed into the Designated List of Existing Substances subject to Standard Registration;
- The annual manufacturing/importing tonnage exceeds 1 ton;
Registration Deadline
Situations of Phase I Registration | Standard Registration Deadline | |
Complete Phase I Registration before 31 Dec. 2019 | Annual tonnage exceeding 1 ton, yet no more than 100 ton; | Before 31 Dec. 2022; |
Annual tonnage exceeding 100 ton; | Before 31 Dec. 2021; | |
Complete Phase I Registration after 1 Jan. 2020 | Annual tonnage exceeding 1 ton, yet no more than 100 ton; | Within 3 years from 1 Jan. of the second year of Phase I Registration; |
Annual tonnage exceeding 100 ton; | Within 2 years from 1 Jan. of the second year of Phase I Registration; | |
The annual tonnage is less than 1 ton when the Phase I Registration is completed | The actual annual tonnage exceeds 1 ton before 31 Dec. 2019; | Before 31 Dec. 2022; |
The actual annual tonnage exceeded 1 ton after 1 Jan. 2020 | Within 3 years from 1 Jan. of the second year of Phase I Registration; | |
Note: 1. If enterprises have cancelled the Phase I registration number, yet applied for Phase I registration for the second time, then the enterprises still have to complete standard registration within the original registration period after obtaining the Phase I registration code; however, if the second application for Phase I registration have not been submitted within the time limit, then the standard registration shall be completed at the time of application;
2. If the annual tonnage of Phase I registration exceeded 100 ton and it is expected that repeated dose toxicity test, reproductive/developmental toxicity test, hazard assessment or exposure assessment cannot be completed within the fixed period, then related enterprises may apply for extension of the registration period to the competent authority before six months of the deadline. The registration period may be extended for one year.
Registration Review and Charges
In accordance with requirements of the Regulations, the competent authority will review the standard registration within 90 working days upon receipt of the application (The competent authority shall notify the registrant if the review period is extended. The number of extensions is limited to one time). If supplementation of registration materials is required, then registrants need to apply for supplementation within 30 working days upon receipt of the notice. If registrants fail to apply for supplementation of registration material, or registrants fail to supplement all the information required after two submissions, then application for registration will be rejected. The review periods shall be recalculated from the date that the competent authority receives the supplementation submitted by the registrant.
The competent authority will charge review fee for existing substances standard registration (50000 NTD per registrant per substance). If the registrant is an academic institution or a medium or a small sized enterprise, then the registrant may enjoy a 25% discount (namely the competent authority will only charge 37500 NTD per registrant per substance).
Registrants may apply for CBI protection (including registrant information, substance identification information, manufacturing/ importing information, use information, etc.). Each item will be charged 12500 NTD. The CBI will be protected for 5 years. Enterprises may also apply for extension of confidentiality period. The maximum confidentiality period is 10 years.
Registration Tonnage and Data Requirements
There are four tonnage bands for existing substance standard registration:
- Level I Standard Registration: Annual manufacturing or importing volume exceeding 1ton, yet no more than 10 ton;
- Level II Standard Registration: Annual manufacturing or importing volume exceeding 10 ton, yet no more than 100 ton;
- Level III Standard Registration: Annual manufacturing or importing volume exceeding 100 ton, yet no more than 1000 ton;
- Level IV Standard Registration: Annual manufacturing or importing volume exceeding 1000 ton;
The registration tonnage shall be determined based on the annual manufacturing or importing volume of Phase I Registration. After completing Phase I Registration, if enterprises like to increase the annual manufacturing/ importing tonnage, then the levels of standard registration will be determined based on the actual annual manufacturing/ importing tonnage.
Following is the data requirements for different levels of standard registration:
Registration Information | Level I | Level II | Level III | Level IV |
1. Basic information of the registrants and identification of the substances | ★ | ★ | ★ | ★ |
2. Substance manufacturing, usage and exposure information | ★ | ★ | ★ | ★ |
3. Hazard classification and labeling | ★ | ★ | ★ | ★ |
4. Safe use information | ★ | ★ | ★ | ★ |
5. Physicochemical property information | ★ | ★ | ★ | ★ |
6. Toxicological data | ★ | ★ | ★ | ★ |
7. Eco-toxicological data | ★ | ★ | ★ | ★ |
8. Hazard assessment information |
| ★ | ★ | ★ |
9. Exposure assessment information |
| ★ | ★ | ★ |
Note: 1. If the existing substances are considered as category I carcinogenic, germ cell mutagenic or reproductive toxic (CMR) substances, then enterprises must submit registration information of the upper level; 2. In terms of Item 5, 6 and 7 registration information, the required data endpoints is different depending on the levels of standard registration.
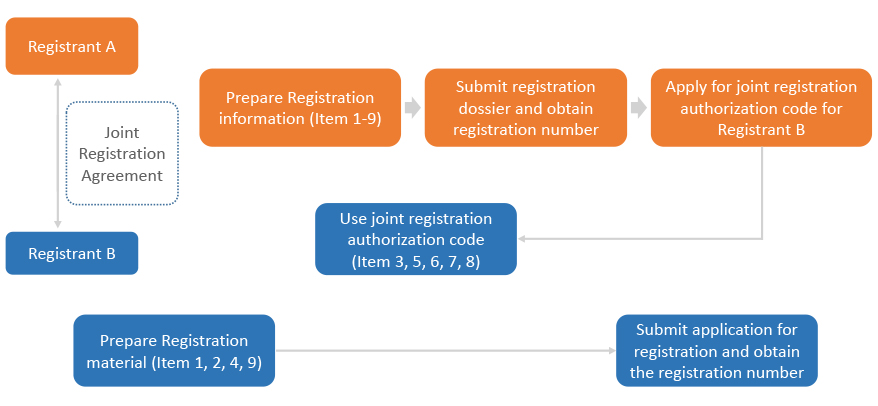
Joint Registration
Registrants of the same existing substance may share the registration information. Registrants may choose single registration or joint registration.
If registrants choose joint registration, then registration information regarding Item 3, 5, 6,7,8 (Hazard classification and labeling, physicochemical property information, toxicological data, eco-toxicological data and hazard assessment information) can be shared. After the first registrant completed the standard registration and obtained the standard registration number, the first registrant shall provide the joint registration authorization code to co-registrants. Co-registrants only need to provide registration information regarding Item 1, 2, 4, 9 (Basic information of the registrations and the substances, substance manufacturing, usage and exposure information, safe use information and exposure assessment information).
Existing Substance Pre-Registration in Taiwan
The revised Toxic Chemical Substances Control Act (TCSCA) in Taiwan was officially promulgated by President Ma Ying-jeou on 11 December 2013.
The English version of the full text of the revised TCSCA (Non-official translation) can be downloaded here free for charge
Regulation Requirement
The revised TCSCA requires enterprises to register new substances 90 days prior to production or importation, pre-register (Phase I Registration) existing substance designated existing substances manufactured or imported above a given quantity.
Pre-registration (Phase I registration)
All manufactured or imported existing substances with tonnage 0.1t/y shall be pre-registered before 31th, March, 2016. Company information, basic substance identification information, produced or imported volume and use information shall be provided via IT system. In order to keep confidential business information (CBI), a manufacturer outside Taiwan may appoint a Taiwan local agent (TPR, Third Party Represent) to finish pre-registration.
Late pre-registration will be applicable after 1st March 2016 for first production and importation after that.
New or Existing
A new substance is defined as a substance is not listed in Taiwan Chemical Substance Inventory (TCSI) published by Authority. More information could be found here.
Registration Type
There are three types of registration: standard registration, simplified registration and small volume registration. Standard registration includes four ties and data requirements increase by degrees.
Annual | PPORD | R&D | CMR+1 | Standard | On-site isolated intermediate | Polymer | PLC |
1000t | St. reg. I | St. reg. IV | St. reg. IV | St. reg. I | Sm. reg. + pre evaluation | ||
100t | St. reg. IV | St. reg. III | |||||
10t | St. reg. III | St. reg. II | |||||
1t | Sim. reg. | St. reg. II | St. reg. I | Sim. reg. | |||
100kg | Sm. reg. | NA | St. reg. I | Sim. reg. or Sm. reg. | Sm. reg. | NA | |
Management of Toxic Chemical Substance
Download the toxic chemical substance inventory here.
The Act also strengthens the management of Class 4 toxic chemical substances. Enterprises are required to declare relevant toxicological information and obtain approval from competent authorities for Class 4 toxic chemical substances prior to handling. The provisions pertaining to chemical registrations have come into force on 11 Dec 2014. CIRS summaries major points of the rules.
The table below shows the definitions of Class 1, 2, 3 & 4 toxic chemical substances and how they are regulated under TCSCA.
Category | Class 1 | Class 2 | Class 3 | Class 4 |
Hazard | Hard to break down in the environment and easily to pollute the environment and endanger human beings health because of the bioaccumulation, bioconcentration, or biotransformation. | Has the ability to cause cancer, infertility, birth defects, genetic mutations, or other chronic diseases. | Human beings health and environment will be endangered immediately after exposure to chemicals. | There is a risk to endanger human beings health and the environment. |
Management | 1. Obtain the permit(manufacture, import or sell toxic substances more than the large scale handling standard) | Toxicological information concerning the toxic chemical substances should be submitted and approved. | ||
Label and SDS | yes | yes | yes | yes |
Note 1: The validity period of the permit, the registration document and approval is 5 years.
Note 2: Only domestic enterprises in Taiwan can apply for handling permits.
SDS and Label under TCSCA
Under revised TCSCA, the handler shall, pursuant to regulations, mark matters related to toxicity and pollution control on Class 1 to Class 4 toxic chemical substance containers, packaging, handling sites, and facilities, and shall keep safety data sheets for the corresponding toxic chemical substances. GHS SDSs and labels are compulsory for Class 1 to Class 4 toxic chemical substances and mixtures containing them.
Penalty
The table summarizes the penalties for failing to register substances under revised TCSCA.
Category | Comments |
Failure to notify new substance; |
|
Failure to register designated existing substance. |
|
Our Service
- General consultancy & training;
- Search and confirm if a substance is new in Taiwan;
- Pre-registration and TPR service;
- Dossier preparation and submission of new substance notification and existing substance registration (full, simplified, small volume);
- Test monitoring/translation of study reports;
- Preparation of SDS and label in compliance with Taiwan GHS;
- Regulatory update monitoring.
About us and contact
We have provided one-stop chemical notification and GHS services for many companies doing business in/with Asia (for example, China, Japan, Korea, Taiwan, Malaysia, and Philippine). We help them find out how their chemicals are regulated in those countries or regions and offer free initial consultations about how to comply. If notification is required, we help them submit chemical registrations (sometimes via our local partners). We also prepare or translate GHS compliant SDS and label in accordance with their national chemical legislation at affordable prices.
If you have any questions about chemical compliance in the Asia-pacific region, please contact: service@cirs-group.com

