The Ministry of Environment has officially announced a draft version of amendment on Presidential decree and Enforcement rule of the Registration and Evaluation, etc. of Chemical Substances Act (K-REACH). The MoE will receive public opinions on this by July 9th.
I. Key Aspect of K-REACH Amendments
1. Main Amendments on Subordinate Ordinance
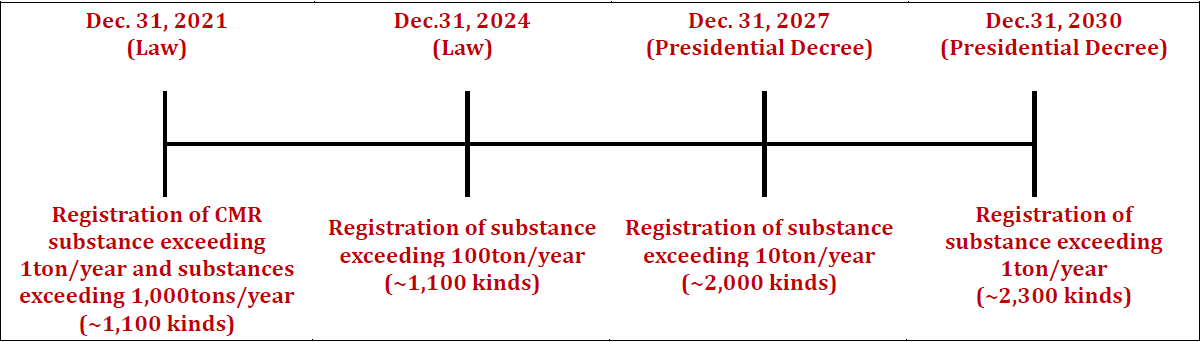
- Registration of imported and manufactured all existing chemicals exceeding 1 ton/year
- Existing chemicals data should be registered by 2030 according to its volume and hazard of chemicals in order to obtain and manage hazardous information of the chemicals circulated in Korea.
- The Presidential decree clearly stipulates that 10 to 100 tons must be registered by 2027 and 1 to 10 tons by 2030.
However, it needs “pre-registration” from January to June 2019 in order to obtain a grace period for each tonnage band of existing chemicals. Following information is required for pre-registration; company information, chemical name, annual manufacturing and importing volume, classification and labeling and use information of chemical.
- Change from registration to notification for new chemicals under 100 kg
Subject | - New chemicals under 100 kg - Substance that has received Low volume exemption(less than 100kg/year) and Low concern polymer exemption under the previous " Toxic Chemical Control Act " |
Submission period | Prior to manufacture or import the substance |
Submission method | Through online IT system(Chemical Information Processing System) |
Authority | The NIER (National Institute of Environment Research) under MOE |
Required information | - Company information (manufacturer, importer or Only representative) - Chemical name - Classification and labelling - Use classification and category (Industrial/professional use, consumer use) - Toxicological data only if the applicant owned |
- Registration based on accumulated total domestic manufacturing/import amount
- Even if the total domestic manufacturing and import volume is not subject to registration by individual companies, it can be designated as substance subject to registration consideration of 1 ton for new chemicals and 10 tons for existing chemicals as a whole.
Category | Based on individual company | Total domestic manufacturing and import amount |
New chemical | 100 kg and above per year | Exceeding 1 ton in total (Even if below 0.1 ton for individual company) |
Existing chemical | 1 ton and above per year | Exceeding 10t in total (Even if below 1 ton for individual |
- Penalty Provisions
- In addition to penalties, fines (less than 5 % of the total sales of the company) are imposed when a substance is imported/manufactured without registration or change registration. It is implemented to redeem economic profits.
- The total sales amount shall be the average annual sales of the three years prior to the year when violated the law. Fine can be adjusted according to the significance, period· number of violations, and profit and liability earned for the offence.
- Amendments to reduce industry’s burden
Simplification of registration dossier for non-hazardous chemicals | There are lots of data to be submitted in terms of tonnage, but for non-hazardous existing chemicals (not having GHS classification or labelling for health and environmental hazards), data corresponding to lower tonnage band (1-10 ton) regardless of its tonnage band are required. But, this simplification will not be applied in case for consumer use |
Transported isolated intermediate(TII): Exempt from data requirements for less than 1,000t of TII. Only data corresponding to lower tonnage band (1-10 ton) should be submitted for over 1,000t of TII.However, it is necessary to submit documents to verify transport or use(s) are under strictly controlled conditions and record the management log. | |
Change of Polymer exemption rule condition | In the case of polymer with less than 10,000 average molecular weight, they shall not be exempted if they are; hazardous chemicals, priority control chemical substance, new chemical remains at 0.1 % or more as non-reactive monomers and it affects on the hazards of polymers |
Improvement to organize Substance Information Exchange Forum (K-SIEF or CICO) | Separate SIEF may be organized if - the use and characteristics of substance is different within CICO member and difficulties in discussing issues in CICO - those who manufacture or import the substance for consumer use |
If the characteristics such as molecular weight, solubility etc. are clearly different for a polymer with the same chemical name, they can form separate SIEFs, considered as different substances. | |
Improved application for exemption of chemical reagent registration | In the case of reagents, it is allowed to submit the application for registration exemption within 30 days after its manufacture and import |
If multiple substances are imported on a product basis, it is allowed to apply for an exemption (including the remaining components) as representative substance of the product. | |
Sharing new chemical evaluation results with the Ministry of Employment and Labor(MOEL) | The MOEL is prosecuting the revision of relevant regulations in consultation with the MOE to replace current hazards·risk assessment of new chemicals under the occupational safety and health administration (OSHA) by registration of new chemical substances under K-REACH in order to avoid duplicate tasks (OSHA pre-announcement of legislation, March 30 2018) |
The MOE also revised regulations for the MOEL to access new chemical registration dossier submitted by companies to the Information Processing System (IT). |
- The transfer of chemical information in supply chain
- The person who supplies the chemical and chemical products should provide the registered chemical substance as well as the hazardous chemicals designated by the MOE and Chemical safety information of hazardous chemicals (registration number, chemical name, hazard and risk information, and safe use information) to downstream users in supply chain.
- In this occasion registration number or notification number may be omitted for new chemicals or unregistered chemicals, but information should be provided if any hazardous chemicals are contained higher than the cut off limit of content.
- Chemical information of CMR substances to be published by the MOE should be provided if its content is higher than the cut off limit in accordance with GHS standard.
- Improving the assessment method of chemicals in NIER
- Under current K-REACH, the NIER is to evaluate all of chemicals submitted. But after the amendment, when evaluating the hazard assessment of existing substances, factors such as amount of domestic manufacture/import, hazard, possible risk to health and environment, etc., are prioritized and reviewed by NIER.
- The existing chemicals to be jointly registered clearly regulates all lead registrants and members of K-SIEF to complete registration or to start evaluation when the grace period expires.
2. Amendment and Enforcement date
- The enforcement ordinance must be passed by the Cabinet meeting, and must go through a process of regulatory review and legislative review after the notice of legislation.
- It is expected that the revision will be completed later this year considering that it usually takes 40 days for legislative announcement, 1 to 2 months for regulatory review, and 1 to 2 months for legislative review.
- However, the amended subordinate statutes will be implemented in Jan 1 2019 along with the Act.
II. Industry's Countermeasure
1. Public opinions are accepted until July 9 2018.
2. Preparation of pre-registration of existing chemicals
- A person who manufactures/imports existing chemical substances must pre-register basic information such as chemical name, manufacture/import amount etc. to ensure its grace period for registration deadline. If the substance is not pre-registered on time, the manufacturing/import, use, and sale of the substance is immediately suspended after July 1 2019, and the grace period to manufacture/import the following substance cannot be granted. Therefore, it is recommended to establish the inventory of manufacturing/import chemicals to prevent omission
3. Measures to provide information about chemicals in supply chain
- Management of relevant information in chemical products is required since supplier should provide chemical information regarding hazardous chemicals as well as registered chemicals as mandatory.
4. The measures to penalty
- Thorough management of manufacturing and importing chemicals within the company is highly required as fines (less than 5 % of the total sales of the company) are imposed when a substance is imported/manufactured without registration or change registration apart from the penalties.
