The CCTV “315” Consumer Rights Evening Party in 2019 exposed two companies which illegally produced sanitary products. They are “Bester” and “Jindeli”, respectively, from Hubei Province and Shandong Province. According to the reporter, these two companies produced sanitary products with the “dirty pulp”. The “dirty pulp” (4000 RMB/ton) were made of the recycled waste sanitary products. They are cheaper than the “clean pulp” (8000 RMB/ton) which were made of wood pulp. These two companies not only illegally gained profits, but also seriously violated the "Hygienic Standards for Disposable Sanitary Products"(GB15979). The Market Regulatory Authority punished them at once, and then thoroughly inspected the city's sanitary products.
As a consequence, more attention has been paid to the production and supervision of disposable sanitary products. In fact, State and local authorities never stop the supervision and administration of disinfection products. They carry out spot checks from time to time. Spot checks are based on several regulations and standards, including the “Measures on the Administration of Sterilizing”, “Measures for Labels and Instructions of Disinfecting Productions”, “Regulations on the Safety Evaluation Report of Disinfecting Products”, “Hygienic Standards for Disposable Sanitary Products” and so on.
In 2016, the National Health and Family Planning Commission deployed the supervision and inspection of disinfection products in 31 provinces (districts, municipalities). The focus of sample check was on wet wipes, hygiene wet wipes, antibacterial and bacteriostatic agents, automated washer and disinfectors, hand antiseptic agents etc. The results are shown Fig 1. The National Supervision Center sampled 102 disinfection products from 42 manufacturing companies. These products were class I high level disinfection products for medical devices. The results indicated that 31 companies had various problems on production conditions. Furthermore, unqualified products are caused by exaggerated or even false claims in label, incomplete information in label, and the absence of hygienic safety evaluation report.
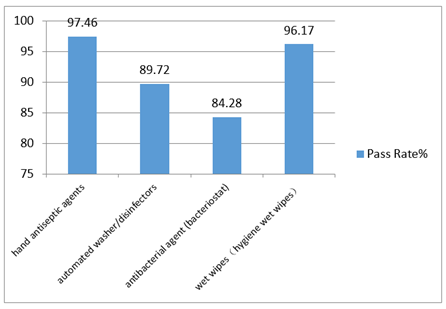
Fig 1. The inspection results of disinfection products in 31 provinces in 2016
In 2017, the supervision was carried out in the form of “random inspection” and was focusing on in course and afterwards stage when compared with previous in advance administration. CIRS sorted out the spot check results of disinfection products in Beijing, Shanghai, Jiangsu, Tianjin, Heilongjiang and Gansu. Details are shown in Fig 2 and Fig 3. The main problems of unqualified products were: the labels didn’t meet the requirements of the “Measures for Labels and Instructions of Disinfecting Productions”, and the microbiological indicators of products were not up to standard. For the manufacturers, there were problems with hygienic license, production conditions and factory inspection reports.
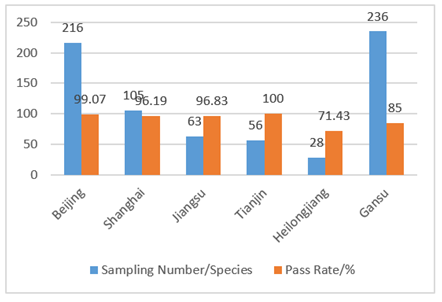
Fig 2. Results of spot check of disinfection products in various cities in 2017
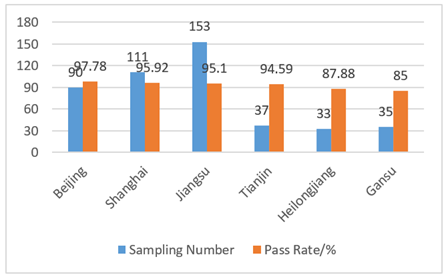
Fig 3. Results of spot check of disinfection products manufacturers in various cities in 2017
In 2018, with the development of the “random inspection and public release across the board”, the National Health and Family Planning Commission emphasized that the focus of spot check was on “84” liquid disinfectant and the sanitary products for adults and babies. Shanghai, Zhejiang, Jiangsu, Anhui and other provinces then conducted related spot checks on disposable sanitary products. Taking Zhejiang province as an example, 295 products were sampled, with a pass rate of 97.96%. Among them, the sanitary products for woman and infants were all qualified. Other unqualified disinfection products still had similar problems as those in 2017 and 2016.
By analyzing the supervision on disinfection products in the past 3 years, CIRS got the following conclusions:
1. For sampling objects, more than 90% spot checks were carried out to the production enterprises, which aimed to prevent poor quality and non-compliance products from the source.
2. For the product types, disposable sanitary products have been the focus of spot checks. There are three reasons:
- As daily necessities, disposable sanitary products have a wide range of circulation. The users involve special sensitive people such as women, babies and the elderly;
- The market demand and the usage of disposable sanitary products increase rapidly. According to statistics, the consumption of disposable sanitary products in China reached 82.94 billion Yuan in 2016;
- Due to fierce market competition, manufacturers choose to improve their market competitiveness by “mass production” and “cost reduction”, which would lead to poor quality and market confusion.
3. When sampling disposable sanitary products, the following two aspects are mainly inspected:
- Label and instruction;
- Hygienic quality indicators.
The inspection for the production enterprises cover the whole production process of disinfection products, which includes:
- Hygienic license
- Label and instruction
- Production monitoring
CIRS also sorted out the problems of poor quality disinfection products and analyzed the unqualified reasons in Table 1.
Table 1. Problems of poor quality disinfection products and the reason analysis
Unqualified items | Details | Reason |
Label and Instruction | 1) There are exaggerated or even false claims in label. It makes consumers mistakenly think that the product has effect in treating disease. | Violate the “Measures on Labels and Instructions of Disinfecting Productions” |
2) The content of active ingredient is not up to standard. | ||
3) The information in label is incomplete, lack of essential information, like the hygienic license, No responsible entity and manufacturer info, trademark name etc. | ||
4) With prohibited content in the label. | ||
5) Hygienic license, the information of production or enterprise and other information are out of date. | ||
6) The uses of the product go beyond the defined scopes. | ||
Hygienic Quality | 1) Without hygienic safety evaluation report or an incomplete hygienic safety evaluation report | Violate the “Regulations on the Safety Evaluation Report of Disinfecting Products” |
3) Hygienic quality test report is unqualified. | ||
Production Monitoring | 1) The acceptance control of raw material is loose, use banned or prohibited substances, like hormones.. | Violate the “Hygienic Standard for Factories Producing Disinfectants” |
2) The layout of the purification workshop is unreasonable, lack of necessary hygienic facilities. | ||
3) Poor production environment and unqualified water. | ||
4) Production batch records and goods-out records are incomplete. | ||
5) There are irregular operations in production and checking. |
4. For imported products, the inspection is only focusing on the circulation process, and will not involve the production process. But, the state and local health commission will carry out spot checks on China responsible entities. For example, 43 imported disinfection products and 30 China responsible entities were sampled in Beijing, in 2017. The contents of spot check were:
- Business license: to check the business scope of the China responsible entity to see if it includes disinfection products, to check the product information in the customs declaration and the certificate of free sale and the product label to see if they are consistent.
- The approval license for new disinfection products: to check if the product in the license conforms to the product sold in the market.
- Hygienic safety evaluation report: to check if the hygienic safety evaluation report of imported disinfection products is complete and qualified or not.
- The quality of imported disinfection products: to check if the product is in validity, and to conduct tests on content, microorganisms index and germicidal efficacy to see if the product complies with related standards.

